Why CO2E?
While fossil fuel derived molecules produce CO2 when burned, we do have the ability to use electricity to convert the CO2 back into fuels with renewable generated electricity from wind, solar, etc. and thus reverse the amount of CO2 in the atmosphere. This is a very creative approach to solving our current issue with regards to CO2 induced climate change. However as air is 400ppm of CO2, the energy we need to upconcentrate this and then upconvert it to something like a fuel will never compete with either using this electricity directly or even using it in a battery (90% vs 40% roundtrip efficiency).
The fundamental principle of why batteries are more efficient than electrolysis is that batteries only change the oxidation state of an element, whereas electrolysis forms covalent bonds. Covalent bonds are great in that they can last for 1000's of years whereas a change in oxidation state in a battery could probably not maintain its charge for more than a couple of years. Covalent bonds also have a much higher energy density than batteries. However in terms of sustainable energy, we are fine with battery stability and the energy density they provide is sufficient for most all of our applications.
Thus why investigate CO2 electrolysis? Both ships and especially airplanes need high energy density fuels. On shorter trips batteries may suffice, however a ship travelling directly from China to Europe would struggle without an energy dense fuel on board. Airplanes are even worse as the weight of the low energy-density batteries adds substantial weight to the plane that will not go empty near the end of the trip the way liquid fuels do. Together airplanes and shipping represent roughly 5% of our greenhouse gases. Still if our round trip efficiency is 40%, maybe 50% with great research, we are still nowhere near batteries and the fossil fuels we use now have the same economic value as batteries. Thus if we need to go to CO2 electrolysis field, we are looking at 2x increase in fuel costs, and as these dominate aviation and shipping costs expect shipping and aviation costs to go up substantially as we go sustainably. There is really no easy solution for this, and not even a moderately hard solution, so we will need to plan accordingly.
As shipping and aviation will be tough and people may try to avoid these, is there anywhere else for CO2 electrolysis? Yes- the organic chemicals industry. Organic chemicals, by definition, need carbon, thus meaning batteries are non-competitors in this field. Furthermore the chemicals industry, dominated by plastics, contributes to 5% of our CO2 emissions. The only competitors for CO2 electrolysis are biomass, H2 electrolysis + thermal catalysis with CO2, and fossil fuel useage + carbon capture. The greatest thing about organic chemicals is that CO2 electrolysis does not need to be the best approach for all chemicals, but if it optimal for just one, it will be a success. As there are hundreds of chemicals that are produced on the kiloton scale there is a high likelyhood we will be succesful somewhere. One important point to note is that the chemicals industry has inherently higher value products than the fuels industry because chemicals need to be both selective and functional. For example while natural gas is cheap/low value, because it is mostly methane with a little ethane and propane, the only reaction these molecules are good at is combusting to CO2.
Comparison to competing technologies
Currently the chemicals industry breaks down long carbon chain fossil fuels / biomass materials start with long chain carbons into small carbon entities (CO, H2, ethylene, propylene), and then builds the desired chemicals from these building blocks. CO2 electrolysis is excellent at producing these small building block materials such as CO, ethylene, ethanol, and propanol, to start with. Thus they eliminate the initial energy and capital consuming oxidation process fossil fuels and biomass goes through. (Realize in fermentation biomass to ethanol, the reaction stochiometry entails one CO2 molecule is produced for every ethanol made).
In terms of CO2 electrolysis competing with hydrogen electrolysis + thermal CO2 catalysis, both are electrolysis based with identical O2 reactions being produced at the anode. Thus the competition becomes whether the additional catalytic barrier energy loss related to reacting CO2 to products (versus H2O to H2) is greater than the energy loss in the thermal catalysis reactions plus the capital costs for those thermal catalysis reactions.
| In terms of
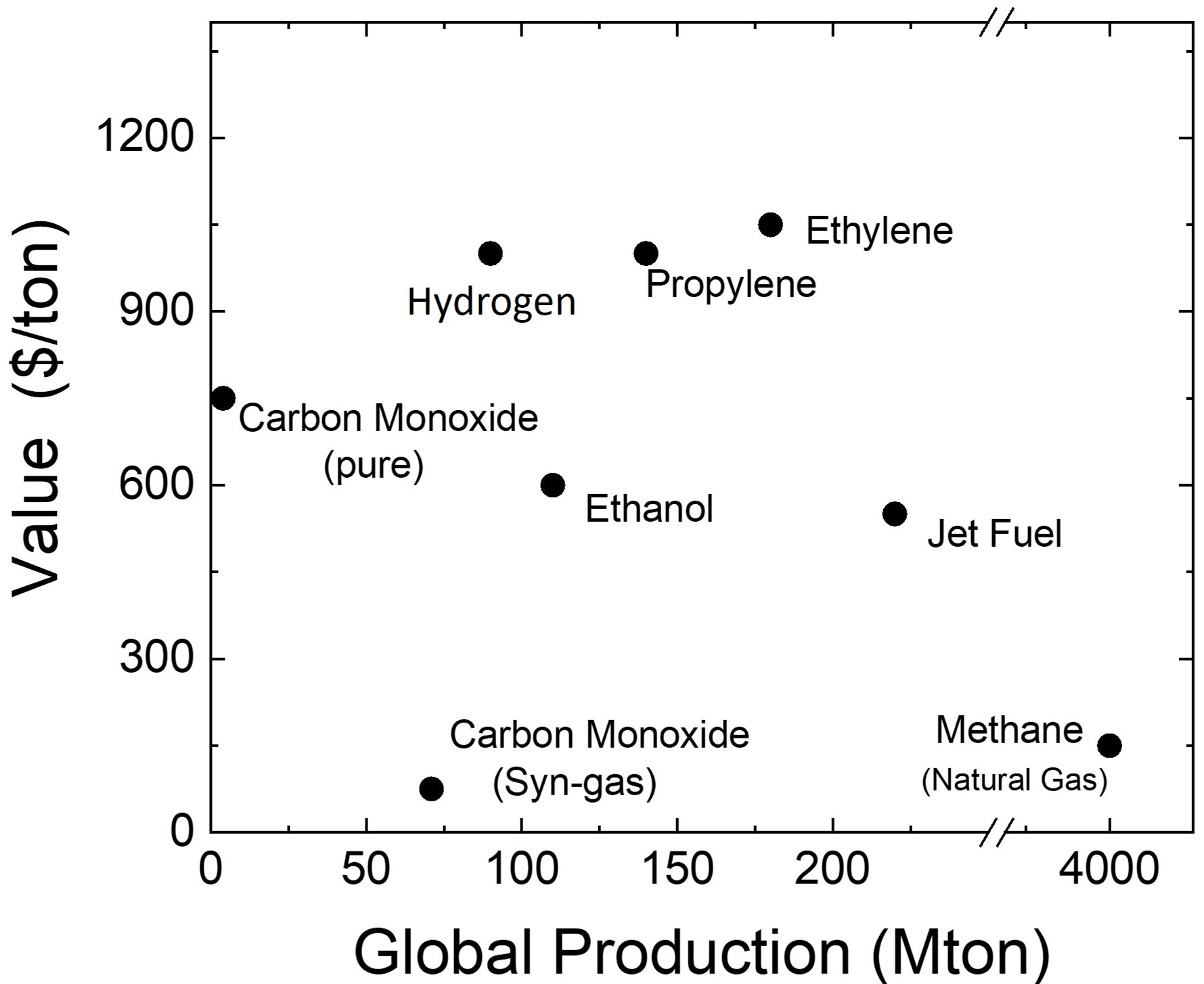
pure economics people often think in terms of what a
chemical costs in $/ton. When we analyze CO2
electrolysis in that aspect, the major products
(carbon monoxide, ethylene and ethanol) seem similar
in value to hydrogen. However this is
what the selling price is, which is not neccesarily
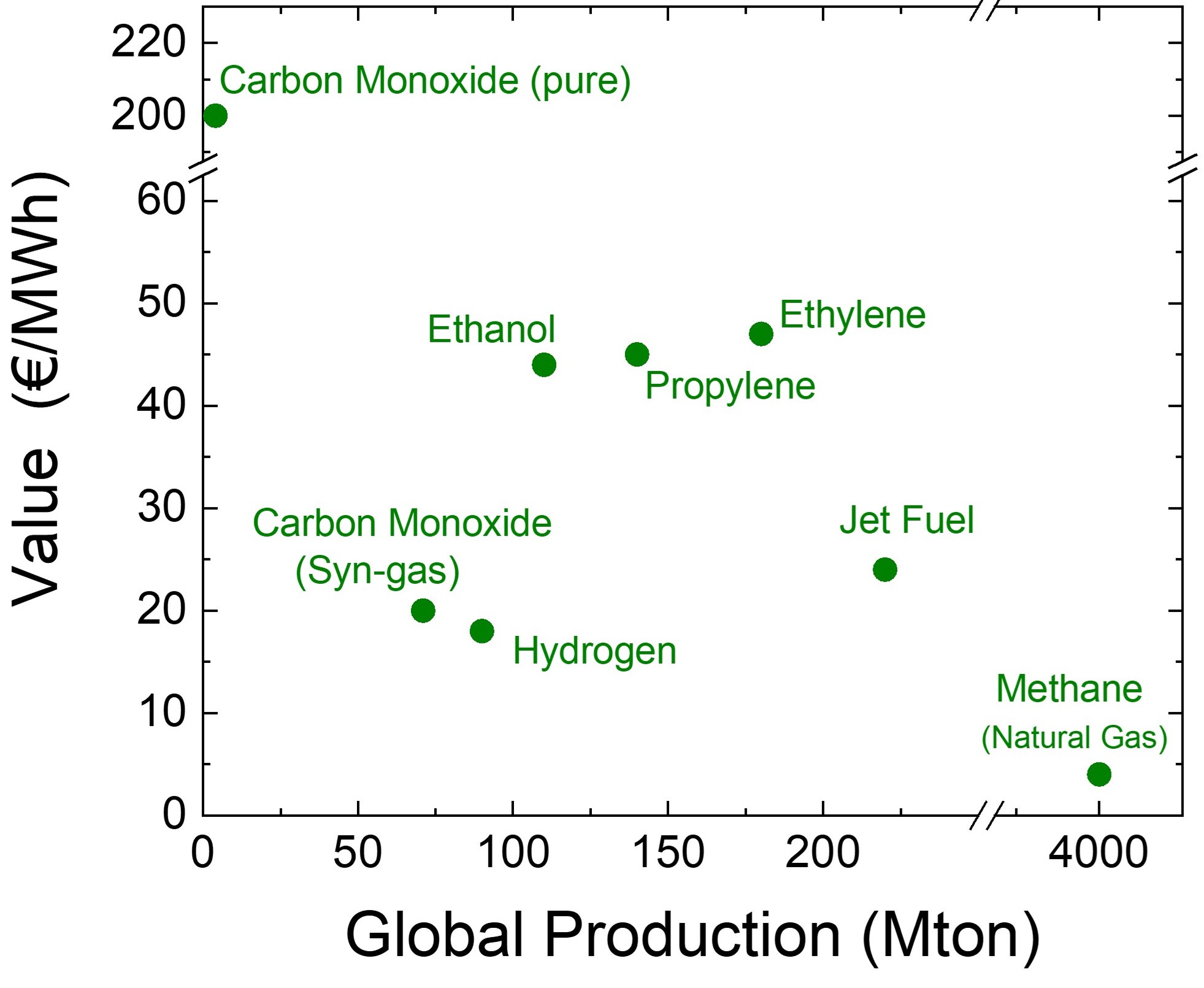
related to the price to produce the species. For something like coal or oil the mass directly relates to how much something costs to dig or pump it out of the ground, thus 'tons' can roughly be thought of as 'cost to produce'. However for electrochemical processes the cost to produce is the number of electrons needed to make the product, thus 'MWh' is a more appropriate cost to produce. Given that both hydrogen and all CO2 electrolysis products have similar thermodynamic potential needed to produce them, they all will operate at similar voltages. It takes 2 electrons to produce hydrogen (H2) and 2 electrons to convert CO2 to carbon monoxide (CO). Thus the power to produce a molecule of each should be similar. However, a molecule of CO is 14 times heavier than a molecule of H2. Given that we sell products based on mass, the value of carbon monoxide will be much more promising via the electrochemical approach. Thus a better way to plot value of products is in terms of $/MWh. That is done on the second chart. When this chart is used it is clear that the CO2 electrolysis products have a much higher value than hydrogen. A practical point to note is that pure CO is much more valuable than syn-gas (i.e. mixture of CO and H2). The reason for this is that much of the cost of pure CO relates to the safety issues related to shipping it. Syn-gas is poduced in refineries and used in refineries eliminating any transport safety issues. Thus on-site CO2 to CO electrolyzers will derive much of their economic benefits due to reducing safety hazards. The issue with CO brings up an important point that CO2 electrolyzers first beachead into commercial market will need to take advantage of their added benefit beyond sustainability. Given the vast amount of chemicals used in today's society, there are plenty of niche markets that CO2 electrolysis can take advantage of. These markets may not impact the world's CO2 problem, but if they are sustainable, economic and can build a supply chain for future electrochemical developments it does not matter. |
 |
 |
A question one may have is whether there will still be CO2 sources once we switch to a sustainable society. The realistic answer is it will take us 50 years to completely get off of fossil fuels. European modeling has denoted that there will be sufficient point source CO2 until 2050. Fields such as cement and fermentation processes intrinsically give off CO2 as part of their chemistry, thus there will always be a certain amount of point sources to pull from.
Great Idea!, Now How Do You Do CO2 Electrolysis?
In the 1980's and 1990's the Hori group tested many different metals as CO2 electrolysis catalysts and showed that materials could be grouped into H2 producing catalysts, CO producing catalysts and formate, all of which are 2 electron reduction processes. Cu was the only material that was able to produce significant quantities of more than 2 electron reduced products.
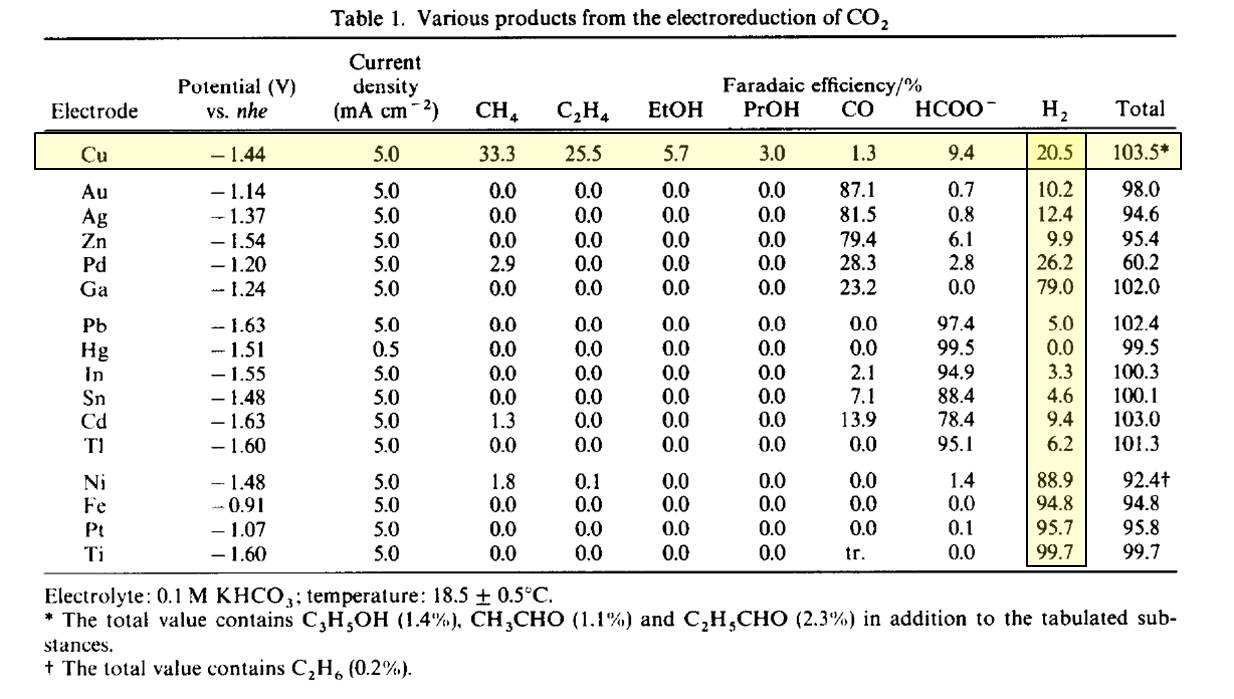
While copper is known to be the best catalyst, there are many ways to modify it to alter its reactivity. Higher surface area, alloying, pH, operating potential and many other parameters are being investigated. From a more fundamental catalysis perspective, the copper has different surface facets, which will bind differently to the CO2, thus effecting the selectivity. Additionally it is well known that the first step when CO2 interacts with copper is that it forms CO, and then reacts further from there. Since there are many catalysts that take CO2 to CO, the reduction of CO on Cu also is quite interesting.
Our Research Focus
Our research is quite diverse and focuses on both copper for CO2 and CO electrolysis, but also gold and silver for CO2 to CO converstion The actual production methods range from simply using copper foils, to sputtering, to size selected nanoparticles, to single crystals.
We test ultra small scale reactions (100 nA/cm2 -1 mA/cm2) in our micro-reactor, research scale reactions in our 3-electrode cells with in-situ gas chromatographs (1 mA/cm2 - 10 mA/cm2), and higher scale reactions in our test cell (10 mA/cm2 - 500 mA/cm2).
| Our microreactor sniffer chip is basically a
porous membrane attached to a mass spectrometer. The
fundamentals of the device was devloped throughout
the last decade in our lab, but more recently have
group members have optimized this for detecting
volatile species in liquid, and we have applied this
to a variety of electrochemical reactions. The
devices have world-class selectivity, which has
allowed for a spin-off company,
Spectro Inlets. These reactors are great for investigating monolayer and submonolayer type reactions, and the time resolution is within about a second whereas a gas chromatogrpah takes 15-20 minutes. |
 |
 |

|
| 3 Electrode Cell Electrochemical Set-up | with in+situ Gas Chomatograph |

Our test cells typically run either 5 cm2 or 25 cm2 devices. We do CO2 electrolysis on the cathode and oxygen evolution on the anode. Operating the system at elevated temperatures is difficult to maintain a consistent temperature, however we operate at 30C, which is enough to mitigate changes in room temperature, but not too hot to create large temperature gradients.

