How does CO2 electrolysis work
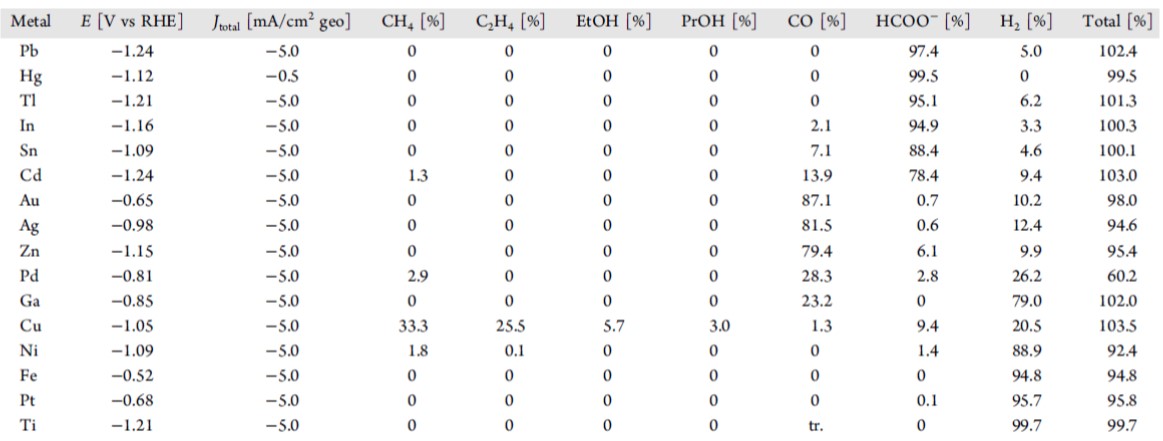
In the 1980's and 1990's the Hori group tested many different metals as CO2 electrolysis catalysts and showed that materials could be grouped into H2 producing catalysts, CO producing catalysts and formate, all of which are 2 electron reduction processes. Cu was the only material that was able to produce significant quantities of more than 2 electron reduced products. The figure on the right groups these materials within the periodic table
 |
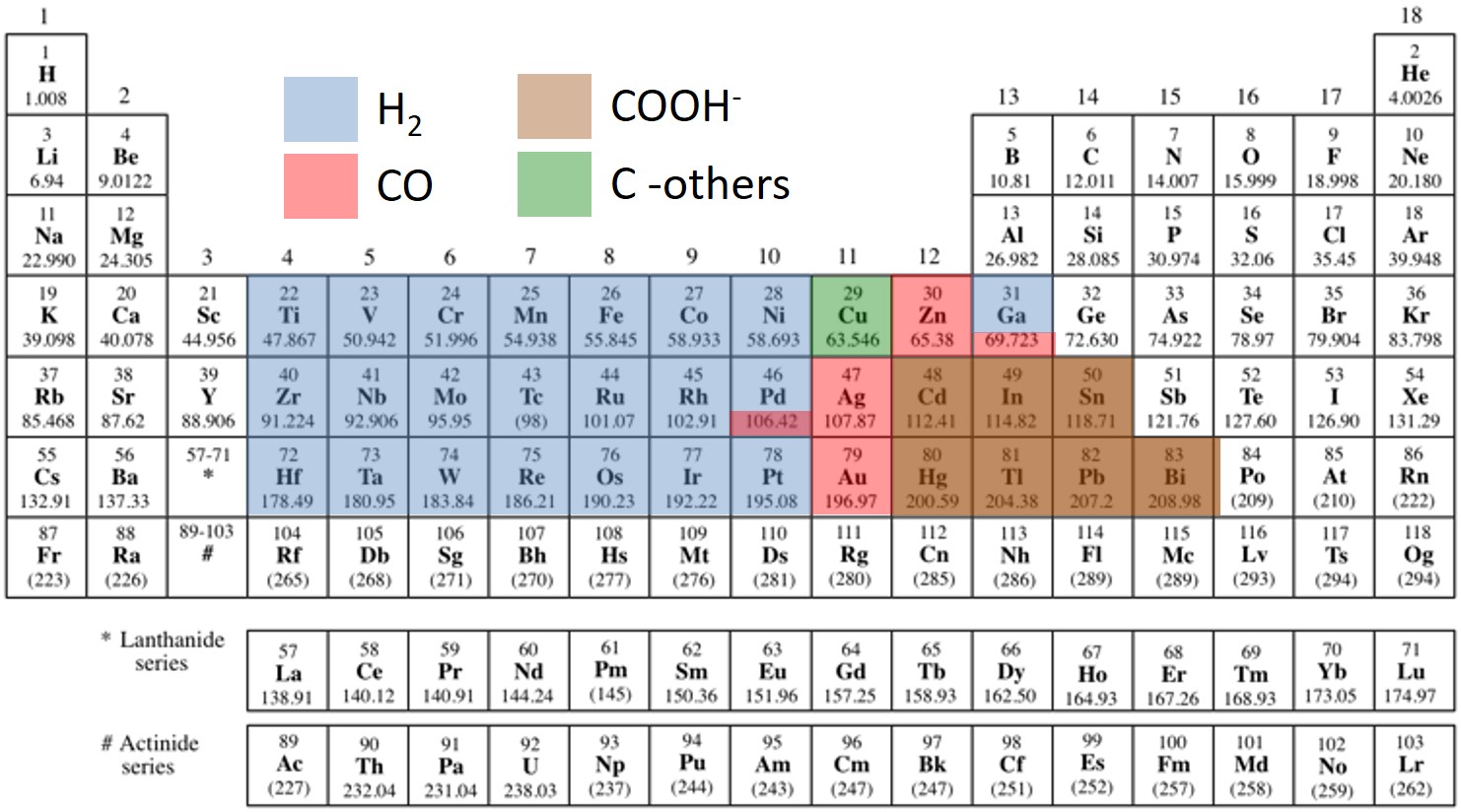 |
From the figure on the right, some clear trends emerge, but the question is why? To understand this we need think about what a catlyst does. A catalyst has species sit on their surface and this reduces their barriers to proceed further in a downhill reaction. (While CO2 reduction is actually an uphill process, once we put enough voltage on the electrode we can make it a downhill reaction). Thus if we are going downhill and we don't want any barrier to our reaction, we want a catalyst that is strong enough to bond a reactant (or an intermediate product), but does not bond it too strong that it gets stuck on the catlayst. This is known as the Sabatier principle. CO2 reduction uses H2O to get hydrogen, thus we need to analyze the binding of carbon, hydrogen and oxygen to a catayst surface. While p-block metals
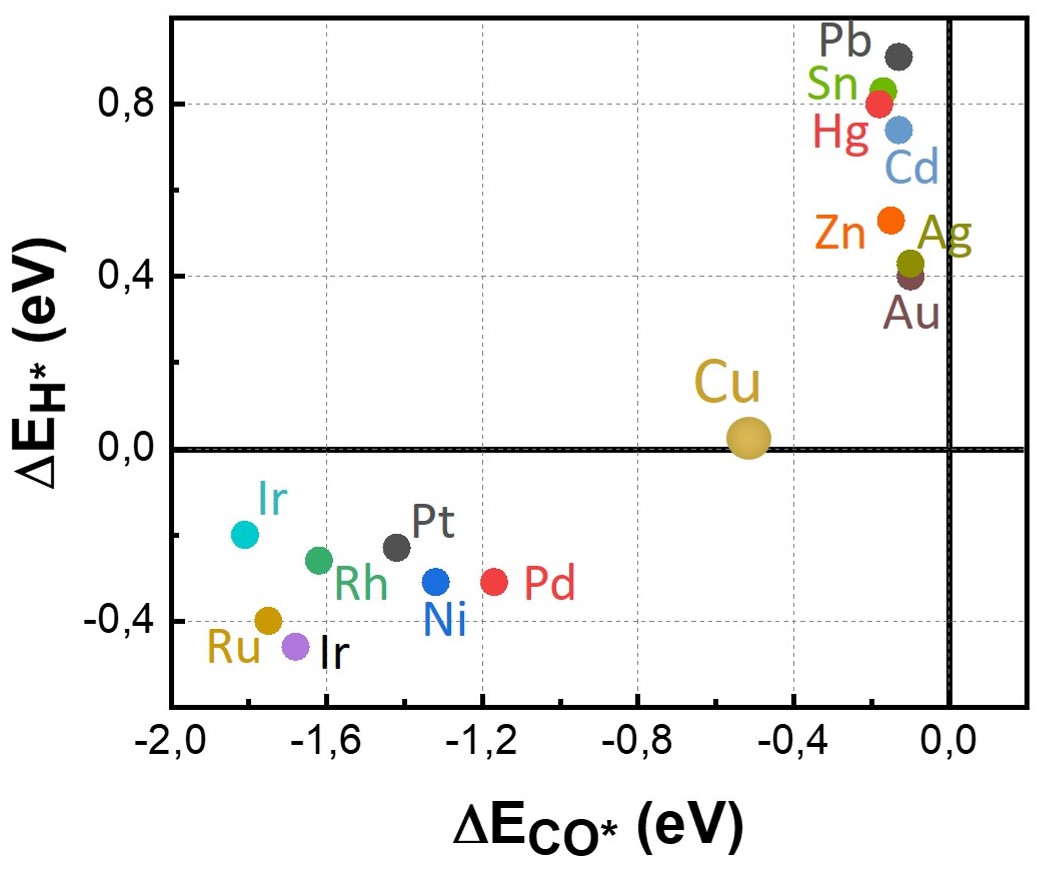 |
While copper is known to be the best catalyst, there are many ways to modify it to alter its reactivity. Higher surface area, alloying, pH, operating potential and many other parameters are being investigated. From a more fundamental catalysis perspective, the copper has different surface facets, which will bind differently to the CO2, thus effecting the selectivity. Additionally it is well known that the first step when CO2 interacts with copper is that it forms CO, and then reacts further from there. Since there are many catalysts that take CO2 to CO, the reduction of CO on Cu also is quite interesting.
Our Research Focus
Our research is quite diverse and focuses on both copper for CO2 and CO reduction, but also gold and silver for CO2 to CO reduction. The actual production methods range from simply using copper foils, to sputtering, to size selected nanoparticles, to single crystals.
 |
|
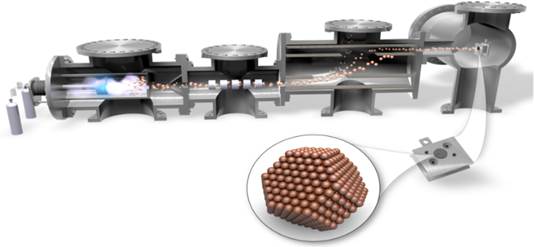 |
| Copper Foil | Sputter deposition of copper | Cluster Source Deposition of Copper |
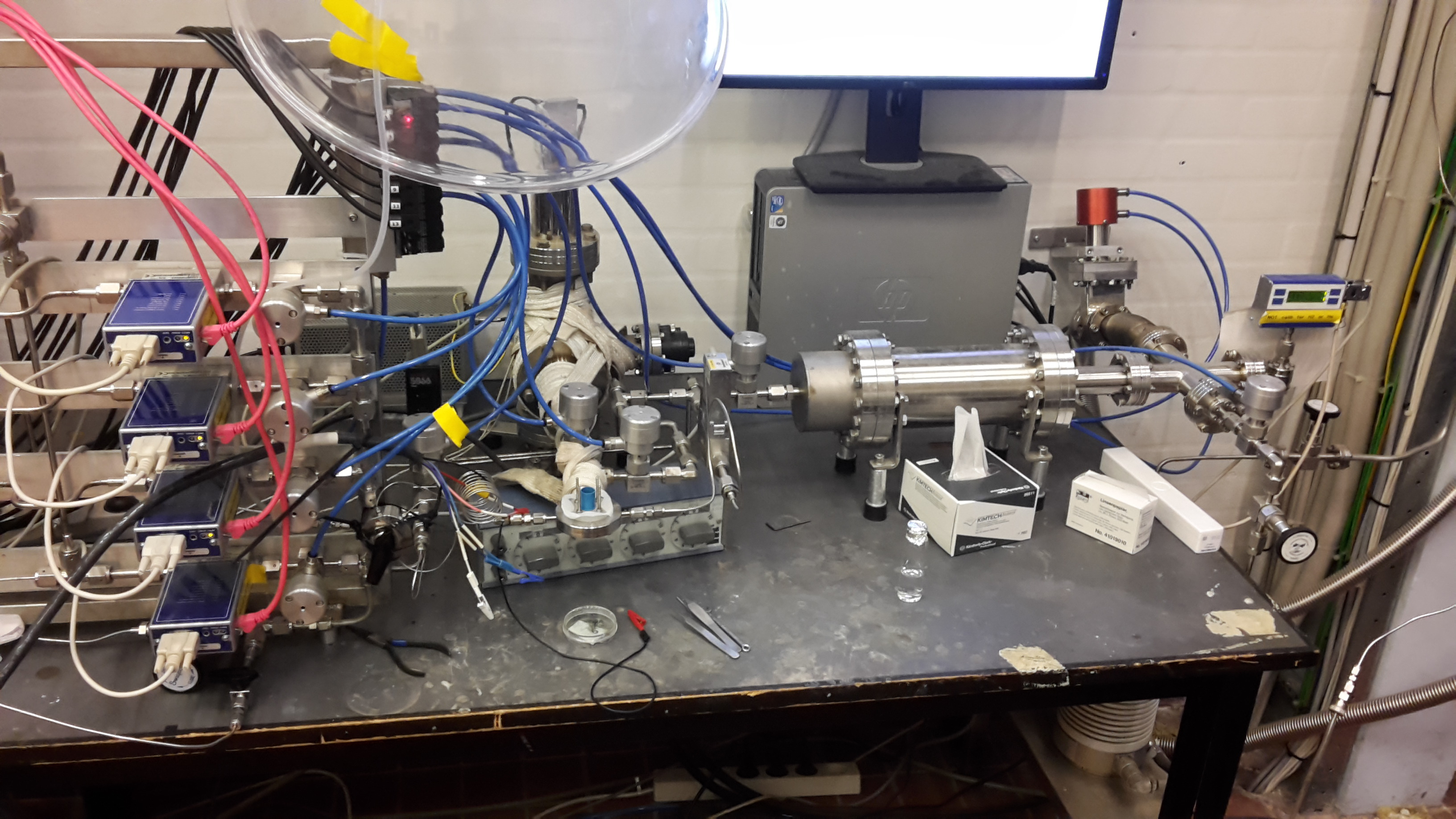
We test ultra small scale reactions (100 nA/cm2 -1 mA/cm2) in our micro-reactor, research scale reactions in our 3-electrode cells with in-situ gas chromatographs (1 mA/cm2 - 10 mA/cm2), and higher scale reactions in our test cell (10 mA/cm2 - 500 mA/cm2).
| Our microreactor sniffere chip is basically a
porous membrane attached to a mass spectrometer. The
fundamentals of the device was devloped throughout
the last decade in our lab, but more recently have
group members have optimized this for detecting
volatile species in liquid, and we have applied this
to a variety of electrochemical reactions. The
devices have world-class selectivity, which has
allowed for a spin-off company,
Spectroinlet. These reactors are great for investigating monolayer and submonolayer type reactions, and the time resolution is within about a second whereas a gas chromatogrpah takes 15-20 minutes. |
 |
 |

|
| 3 Electrode Cell Electrochemical Set-up | with in+situ Gas Chomatograph |

Our test cells typically run either 5 cm2 or 25 cm2 devices. We do CO2 reduction on the cathode and oxygen evolution on the anode. Operating the system at elevated temperatures is difficult to maintain a consistent temperature, however we operate at 30C, which is enough to mitigate changes in room temperature, but not too hot to create large temperature gradients.

