Why Partial Oxidation?
The electrification of the world has impacted many different areas in society, but one of the areas that have yet to take advantage of the fundamental benefits of electricity is the chemicals industry. Much of the chemical industry still uses reactions that were created last century and needs both high temperature and high pressure to be effective which increases the cost, limits the flexibility, and needs extensive safety procedures to operate.
Electrochemical processes actually thermodynamically favor low temperatures and can be done at low pressures thus allowing for a low cost highly flexible approach to producing chemicals. One of the most immediate ways electrochemical reactions can help the chemicals industry is resolving the issue related to gaseous products. One of the major issues in drilling for fossil fuels is that it is hard and expensive to transfer gases. These gases are typically short chain hydrocarbons (C1-C4). By partially oxidizing these gases we can add a polar oxygen group, and just like water this leads to hydrogen bonding, thus making the molecules favor sticking together, and thus becoming a liquid. Methane to methanol is a perfect example of how partial oxidation of a cheap gas (150$/ton) can be converted to a easily transportable liquid in methanol that is actually a higher value product (380$/ton). Furthermore methane to methanol is downhill reaction, thus thermodynamically you harvest energy from this reaction via a fuel cell or other type of device. Thus this process has the potential to make money, and produce electricity.
While electricity will soon be dominated by sustainable energy sources such as wind, solar, and hydroelectric, it may same strange that we are investigating this process to help the fossil fuels industry. However in the chemicals industry we need short chain carbons as precursors for almost all of our plastics. (If used properly plastics are quite environmentally benefical because their light weight reduces transportation costs in comparison to their steel/metal alternatives.) Currently there is not an efficient way to produce these plastic precursors in a sustainable manner. Potential competing sustainable approaches are electrochemical CO2 reduction and bio-based plastics. Bio based plastics start from large chain carbons, and thus need to be partially oxidized as well (now being done using high temp, high pressure procedures), thus some of the breakthroughs in partial oxidation of simple fossil fuels could be translated to the more complex bio based materials. If electrochemcial CO2 reduction use combusted natural gas as a CO2 source, why not just start with the natural gas to begin with? While this electrochemical partial oxidation approach does use fossil fuels, we are not burning them, thus this should not contribute to CO2 in the atmosphere. Most plastics get buried in a landfill, thus to a certain extent we are taking carbon out of the ground, using it, and then putting it back into the ground.
Our Research- Propylene Oxidation
While methane probably has the biggest potential, it is extremely hard to partially oxidize methane without completely oxidizing it to CO2. So our first moelcule we have decided to investigate is propylene oxidation. There has been little research done on proylene, but the research that has been done has shown that proylene can be oxidized via one of 2 paths as shown below.
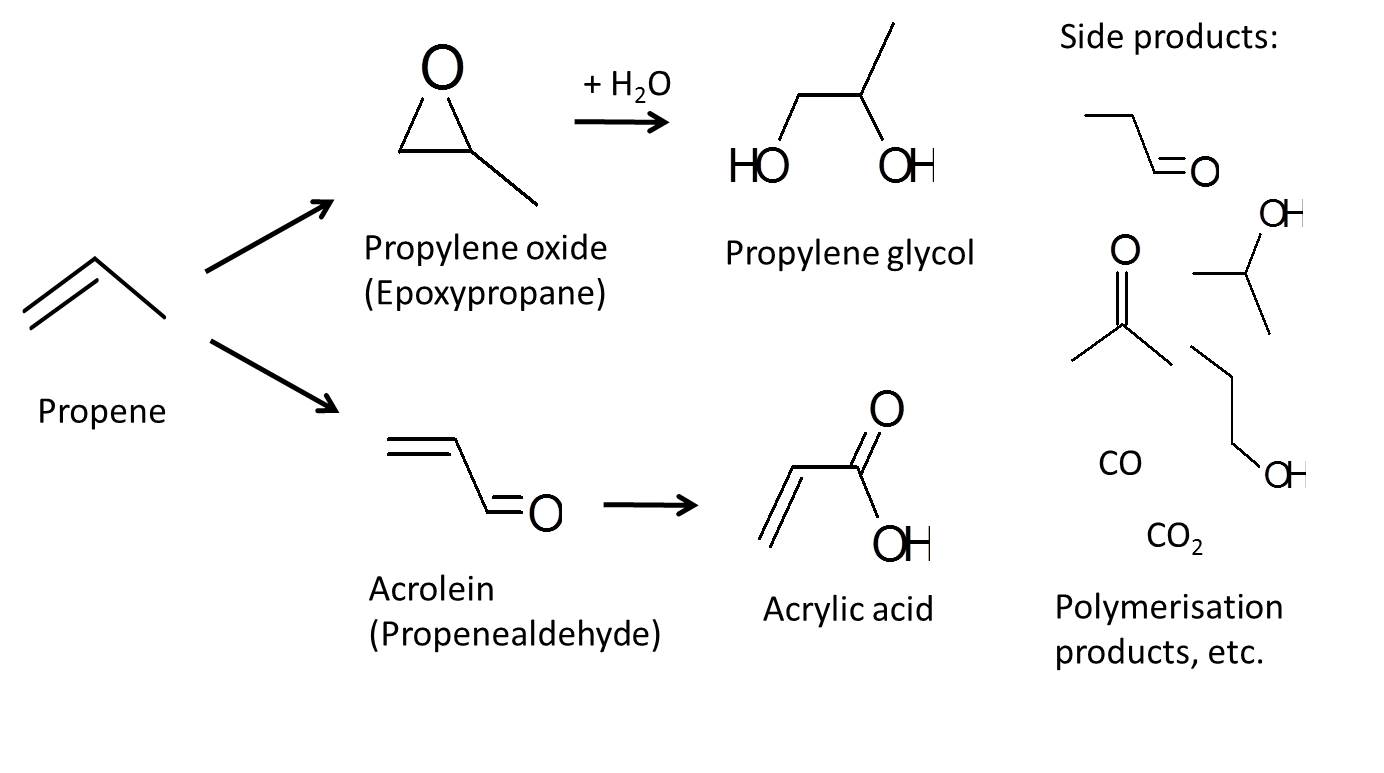
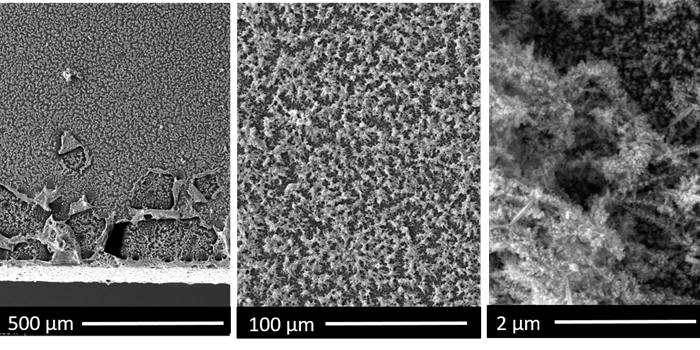
We have designed a specilized catalyst (SEM images shown below) and now can repeat and understand this reaction. We are also working with theoreticians from the Rossmeisl group at Copenhagen University and our results are in agreement with their calculations We have yet to publish our results, however we are making very significant breakthroughs and look to publish our results in the near future. After that time, this page can explain our research in a little more depth.